


Need to contact us quickly? Send us an email by
Clicking Here.





View the largest gallery of high-end and
Monster rainbow toned Morgan dollars, an informational guide
on how to tell natural vs. artificial toning, learn to price
toned Morgans, view auction results of rainbow Morgans, and
view what characteristics to look for in high-end toned
rainbow Morgan dollars.






Lost? Find your way with the sitemap with links to all
the pages on this website. Sitemap


Here you can find interesting articles, videos, and
research tools for coins and currency. We are constantly
adding more links and articles to enlighten collectors.
Click Here


Click here for a how-to
guide for JhonECash.com. The site is very user friendly and
you may not even need to use this page but if you do, it's
right here waiting for you.


Click Here for answers to
ALL of your questions regarding Payment, Shipping,
How to Order, How to Use the Website, and More...




|
 |
Several articles have been devoted in the
past to a discussion of the thin film interference
phenomenon, which is responsible for the colorful toning we
see on silver coins. I would like to delve more deeply into
the physics, to see if we can develop a model for
understanding and predicting the actual "standard
progression" of colors that we observe on silver coins.
For example, why does toning start out light gold? Why does
it then progress to gold, amber, russet, burgundy, cobalt
blue, light blue, lemon yellow, orange, red, magenta, blue,
blue-green, emerald green, and so on. Why can't a coin have
gold toning that progresses to green, or some other color,
rather than following the "standard progression?"
It turns out that once you understand the thin film
interference mechanism, it is possible to model the colors
that we actually observe on the coins. As has been shown
before, the light reflecting off the surface of a toned coin
looks something like this:
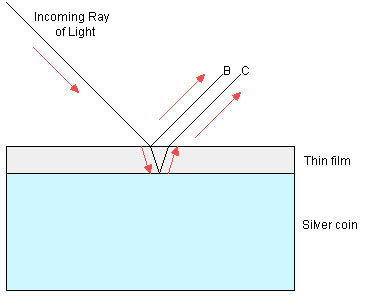
Some of the incoming light reflects off the
upper surface of the toning layer, and exits as ray B.
However, a portion of the light travels through the thin
film layer, and then reflects off the denser metal surface
of the coin underneath the toning layer, and exits as ray C.
Notice that the light in ray C has to travel a longer
distance than ray B. The extra distance (d) traveled is
approximately twice the thickness of the toning layer (t).
[More accurately, d=2t/sin(x), where x is the angle of
incidence of the light hitting the coin's surface below the
toning. For light coming in at a direct perpendicular to the
surface, x=90°, sin(x)=1, and d=2t.]
Because ray C travels the extra distance, the emerging
reflected light of ray C is phase-shifted relative to ray B.
If the extra distance traveled by ray C is precisely equal
to the wavelength of the light (or a multiple thereof), then
the two re-united beams B and C will be in phase, and there
will be constructive interference, or reinforcement, of the
luminosity at that wavelength. If the extra distance d is
precisely equal to one-half the wavelength of the light (or
an odd multiple thereof), then the two re-united beams will
be precisely out of phase, and there will be destructive
interference, or cancellation, of the light at that
wavelength. For intermediate phase-shifts, there will be
moderate cancellation or reinforcement.
(I am leaving out more details, such as one-half wavelength
phase shifts due to differences in the index of refraction
of air vs. the toning layer.)
So what we have so far, is that for a given thickness of
toning layer, and a given wavelength of light, there will be
either constructive interference (reinforcement), or
destructive interference (cancellation) of the light as a
result of the toning layer. As the toning layer thickness
increases, the beams B and C go in and out of phase.
The next step is to understand how we perceive light.
Visible light covers a spectrum of wavelengths from about
400 nanometers (nm) for violet to about 700 nm for deepest
red. A rainbow occurs when full-spectrum light (i.e. light
containing all wavelengths in the visible spectrum) is
refracted by a prism, and separated out into all the
different wavelengths. For each color in the spectrum, there
is a corresponding wavelength of light. However, the way we
perceive light is peculiar to the design of our retina, with
its "rods" and "cones" (the photoreceptors). The cones
perceive color, and there are three types of cones in the
human eye. Each one has a peak sensitivity at a particular
wavelength:
The three types of cones are B=blue (peak sensitivity at
about 440 nm), G=green (535 nm), and R=red (575 nm). The
peak sensitivity wavelengths vary in different sources and
papers on the subject, but you get the idea.
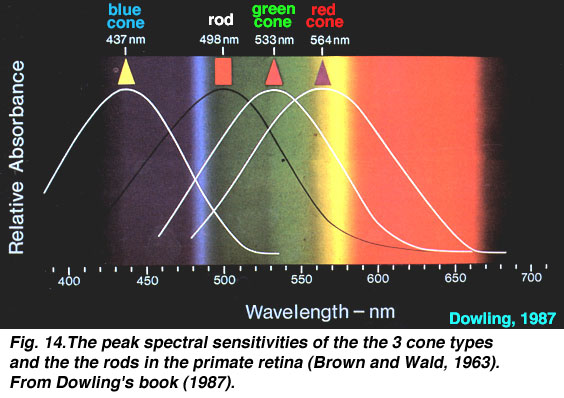
So for example, when our eye sees yellow
light, we know it is yellow because of the way our red and
green cones respond to it, and our brain translates that
into a perception of yellow. The peculiar consequence of our
having three specific cone receptors, rather than one that
covers the entire spectrum, is that you can "trick" the
human eye. If the incoming light contains only two
wavelengths - red and green - the eye will perceive it as
yellow, just as though the incoming light were at the
wavelength for yellow. So I write: G+R=Y. Similarly, blue
light plus green light will be perceived as cyan (B+G=C),
and blue light plus red light will be perceived as magenta (B+R=M).
The combination of all three is perceived as white: B+G+R=W.
Because of this, if you take full-spectrum light, and remove
all the blue wavelengths, that means only the red and green
cones can respond, and the eye sees yellow. This is called
the "subtractive" property of light, which refers not to the
light itself, but the way we perceive it. Yellow paint, for
example, actually looks yellow because it contains pigments
that absorb blue light. We can represent this algebraically
as follows: W-B = (B+G+R)-B = G+R = Y
So, if the toning layer has a thickness that results in the
cancellation of blue light, then the coin will appear
yellow. Depending on which wavelengths get destructively
cancelled or constructively reinforced at a particular
toning layer thickness, the exit light will have some
combination of components of light that appears white (R+G+B),
yellow (R+G), cyan (B+G), magenta (B+R), blue (B), green
(G), and red (R).
Example: if the incoming light has 4 units each of B, G, R;
but the exit light after interference has 3 units B, 2 units
G, and 1 unit R, what will we see? We will see a combination
of one unit white (B+G+R), one unit cyan (B+G), and one unit
blue (B). The result will be a light cyan-blue.
If the exit light contains four units B, five units G, four
units R, we will see four units white (B+G+R), plus one
leftover units of green. This will give us a bright light
green.
If the exit light contains one unit B, five units G, one
unit R, we will see one unit white (B+G+R), plus four
leftover units of green. This will give us a stronger deeper
(less bright) green.
In this way, I can model and predict the color of the exit
light. [For the technical among you, the model depends on
choosing the right peak wavelengths to represent blue, green
and red as seen by our cone photoreceptors. There is some
latitude in the numbers chosen, particularly for red; I'm
not sure whether the peak sensitivity of 575nm is the right
choice, or perhaps a higher number up to 660nm. For our
purposes I am assuming the angle of incidence "x" equals
90°, and I am ignoring the half-wavelength shift off the
upper surface due to the refractive index change, which
actually would imply that light gold toning becomes visible
at even lower layer thicknesses.] Here then is a sample
spreadsheet:
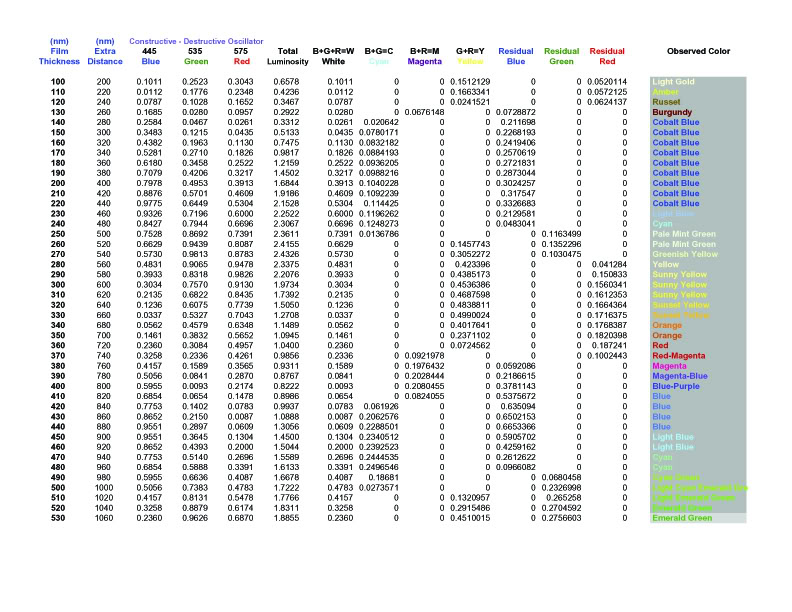
(Click on the picture to view the full size non blurry
example - Opens in new window)
It still requires some tweaking, but this
approach is on the right track, and basically predicts what
we see on the coins. So for example, why does toning start
out in the gold-amber category, before we see any blue?
Because blue light has the shortest wavelength of the three
cones, at 440 nm. That means the phase shift to cancel blue
light is about 220 nm, which requires a toning layer of
about 110 nm (ignoring the angle x mentioned above). So the
very thinnest toning layers cancel blue before they cancel
anything else, and when you cancel blue, you are left with
R+G=Y, so toning always begins with faint yellow (gold),
deepening to amber, russet, and burgundy, before we suddenly
get blue, as predicted in the spreadsheet.
This is not quite complete or perfect, but I hope it helps
to understand the origin of the magnificent colors we see on
toned silver coins, such as this one:

(Photography by Brandon Kelley)
Arguments and Rebuttals:
<<what is this thin film on
some silver coins and not others?
is it part of the minting process, or acquired by the coin
later?>>
The thin film we are talking about here is a layer of
molecular compounds that form naturally at the coin's
surface over time. Silver coins are typically struck from an
alloy of composition 90% silver - 10% copper. Both silver
and copper are somewhat reactive with both oxygen and
sulfur. As a result, under natural storage conditions
(unless the coin is stored in a vacuum or a completely inert
environment such as a helium or argon atmosphere), a layer
of "patina" or "toning" will form at the surface of the
coin, comprising molecules of silver sulfide, copper oxide,
etc.
Anyone who has owned silver or silver-plated housewares will
know that eventually they begin to show color over time.
Therefore most silver ends up getting dipped or polished
periodically to keep it bright and shiny. The same is true
of silver coins. There are some exceptions; if a coin is
stored in an environment that is non-reactive, it may stay
bright over time.
This thin-film layer of toning molecules has a thickness. If
the layer forms uniformly over the whole surface of the
coin, the color will then appear similarly uniform. In an
old-time coin album, for example a Wayte Raymond cardboard
coin board, the perimeter of the coin is in contact with the
cardboard, which may contain elevated levels of sulfur. As
the cardboard outgasses slowly over a period of months and
years, the coin acquires toning from the perimeter inward.
The result is peripheral or target toning, with the thickest
part of the toning layer at the perimeter of the coin. The
coin will exhibit a color progression from the center (least
toned, thinner toning layer) to the perimeter (most toned,
thicker toning layer). The Gobrecht dollar I use as my
avatar is an example. The light blue at the extreme
perimeter actually represents the thickest toning layer
(exactly as predicted by my model) even though it looks
lighter in color.
With Morgan dollars, there was a unique opportunity for
incredible multi-colored banded rainbow toning to develop.
The reason is that, because of politics and history, many
many thousands were stored in sealed bank vaults,
undisturbed for decades in the original canvas Mint bags
(1000 per bag). These storage conditions allowed some of the
coins in these bags (the ones at the surface, near to or in
contact with the Mint bag fabric) to acquire a thin film
toning layer extremely slowly, over a period of many years,
due to an almost negligible air flow in the vaults. A toning
layer so acquired, extremely slowly, can be quite stable
(for reasons of inorganic chemistry more complex than I will
tackle here). Due to a very slight diffusion of gases from
the outside of the Mint bags to the inside, the toning took
the form of a very thin film with gradations in the
thickness across the surface of the coin, rather than
uniform thickness.
<<Given the explaination
above, would the coin you posted (I quoted here) then have a
multi-level "thin film) profile if the coin were viewed
along the plane of the coins surface? Somewhat of a mountain
range profile? Is that what explains the multiple colours or
is it all the same depth of film and the colour progression
is based on angle of light? >>
It is the gradations of thickness that produce the observed
color progression. On the Morgan dollar that I posted above,
the thinnest part of the toning layer is at the top of the
coin, at the BU of PLURIBUS. The thickest toning layer are
the small dark patches between the stars and the rim at 7
o'clock. Viewed in profile, the toning layer would be a
slightly inclined plane, rather than a mountain range with
ups and downs. Rotating the coin in light shifts the
perceived colors only slightly; the angle of incoming light
seems to have less of an effect than the thickness of the
toning layer. (I'm sure one could do experiments though, in
which a very controlled light source could be used,
demonstrating the relevance of the angle of incidence of the
light.)
A coin whose toning is more irregular in profile ("mountain
range") would have similarly wild toning, like this example:

A coin with a uniform toning layer, whose
color is based on the thickness of that layer, does not take
on additional colors by rotating in the light. Here is an
example showing only slight gradation towards the perimeter:

If I tilt this coin in the light, I cannot
get the colors to change to blue etc. Again, the reasons for
this are complex, as one might reasonably expect the
changing angles to affect the extra distance travelled by
ray C, and therefore the perceived color of the exit light.
Part of the explanation may be that the color is caused not
only by the thin film interference, but also by selective
absorption in the toning layer itself. This would imply, for
example, that if the toning layer comprised different
molecules, the colors would be affected. For example, a
sulfur-rich toning layer would appear different from an
oxygen-rich toning layer of the same thickness. And this is
in fact true; silver sulfide yields more colorful toning
than a film of oxides. But mostly it is about the thickness
of the toning layer and the interference phenomenon: the
film layer is too thin to get much red-green cancellation,
no matter what angle the light comes in at.
Incidentally, the coins closer to the center of a stored
Mint bag stayed asbolutely pristine and untoned, as any
reactive gases such as oxygen or sulfur were absorbed by the
outer coins in the bag, which therefore acted as a "getter."
This is why so many thousands of "brilliant" or "white"
coins came out of Mint bags. This in turn fueled an appetite
to collect Morgans that are untoned, "blast white," as that
is what was primarily available. However, once out of the
bags, these coins would begin to tone over time. To feed the
marketplace for white coins, it became common practice to
dip Morgans en masse. Even Mint bags containing beautiful
rainbow-toned coins were sometimes dumped wholesale into
bathtubs filled with dipping solutions. In today's market,
the preference for white Morgans still remains, although I
am doing my best to increase awareness and appreciation of
naturally and beautifully toned Morgans, which are more in
line with our collective preferences for older classic
silver type coins.
The mathematical model in the spreadsheet shown above begins
to explain how such colors are in fact naturally possible.
That's the amazing fact of these coins, that nature created
this intense and amazing palette, with little help from us.
Artificial toning would imply the use of heat or chemistry
to accelerate or otherwise alter the reactive process, but
coins like these Morgans cannot quite be reproduced in even
the most clever coin doctor's lab. Look at hundreds or
thousands of toned Morgans, and you will gain an
appreciation of what is possible by natural processes.
<<If I get this (even a little), are you
saying that a coin with multiple colors has a toning layer
of varying thickness, and it is the change in the extra
distance traveled by the reflected light penetrating the
film which phase shifts and ultimately causes the colorful
look?
Is this also why, as we tilt a coin in hand, we see new (or
maybe enhanced) colors and a change in luminosity?
Or maybe it's the phase shifting of many rays of light
because some only partly penetrate the toning layer before
reflecting? Does that happen?
Or maybe it's due to that intermediate phase shifting thing.
Or maybe I don't get this even a little.>>
YES to to the first question; it is variations in the
thickness of the (extremely thin) toning layer, and the
change in the extra distance traveled (and therefore the
degree of phase-shifting) that causes the interference
phenomenon.
YES to the second question, but the "cartwheel" effect is
also due to what we call Mint luster, a quality of the
surface finish on pristine coins that results from the metal
flow during striking.
Probably NO to light penetrating the toning layer to a
partial depth, and reflecting back before it reaches the
coin's surface.
<<Toning occurs on a
silver surface due to the accumulation of a film( various
sources) which are seen as different colors, due to the
thickness of the film.
The colors progress, with the addition of more film.
Right?>>
Yes, that's basically right. Once the film
gets too thick, the toning will simply appear dark or black,
as it also absorbs more light, so the total luminosity of
the reflected light declines, and there's not enough at any
wavelength to trigger our perception of color.
<<Is the toning the affect
of certain chemicals from the environment interacting with
the copper in the silver. Producing a film that's part metal
and part chemical? >>
It is true that the toning is affected by (in fact caused
by) its environment. And it is true that some environments
will not produce all the colors. But it is not true that
this happens "no matter how thick or thin the toning
develops." If you can get a thin film of toning to develop,
in the range of 100 to 600 nanometers' thickness, then you
will see the corresponding colors produced by this
phenomenon. The cases where it doesn't happen are the cases
where you don't get that thin film; either
a) The toning conditions are so non-reactive that you don't
get a toning layer at all; or
b) The toning conditions are so reactive that the thickness
of the toning rapidly blows past the upper end of the range
where this effect occurs, so the toning just progresses to
dark and unattractive.
Also, if the coin's underlying surfaces are not reflective,
so the luminosity of the total reflected light is too low.
This is why you won't typically see banded rainbow toning on
circulated coins, although if the toning environment is
sulfurous enough, you will still get colorful toning, such
as on the circulated early silver type coins from the
Queller collection. Circulated coins, unless dipped, also
may have a protective layer of oils on the surface that
prevent the formation of oxides and sulfides, giving a
dulled and colorless appearance.
Once you get well beyond the "thin film" range, then the
inherent optical properties of the toning layer itself
become relevant. Every chemical compound has a "color"
caused by the way it absorbs and reflects light. So in this
respect, what leo is saying has some truth to it, for
thicker toning layers. For example, we all know that heavy
copper oxides can appear green or cyan. This has nothing to
do with thin film interference, but rather with the fact
that this particular compound absorbs red light, thus
allowing blue+green=cyan light to reflect. A suitably thin
film of copper oxide, however, can still produce all the
colors of the thin film interference progression.
<<Your explanation makes
sense in the case of coherent light, however we are not
viewing the coins under laser light. Natural light has no
coherence, therefore the incident rays are of all phases. So
how is it that cancellation can occur when incident and
reflected rays have a random phase relationship?>>
that's part of the nature of light, that it
can behave statistically like a wave function despite also
comprising individual photons. For example, the two-slit
experiment provides a wave-like interference effect without
having to use coherent laser light. So the light behaves as
a statistical aggregate of individual waves. Another way to
look at it is that each incident ray is divided, with a
portion bouncing off the upper surface, and the balance
traveling the extra distance. In that case, the interference
will occur. You will say, "but that's impossible, you can't
divide the individual waves." Yet light does behave that
way; on the quantum level, the behavior somehow adjusts to
produce these wave-like effects on a macro basis. The
wave-particle duality is one of the great mysteries of
quantum mechanics - I have my own theories, but not for this
forum !!
It's not a great explanation, but the best I can offer
without brushing away more cerebral cobwebs.
<<I have always wanted to
do a controlled experiment to determine if increasing toning
on a silver coin adds weight to the silver coin or not.
Have you or anyone else ever attempted to explore or test
this?>>
In theory the toning adds to the mass of the coin, because
it represents reaction of the metal surface with ambient
gases. So when a silver sulfide or copper oxide molecule is
formed, the atom of silver or copper remains on the coin,
but is now joined by sulfur or oxygen from the atmosphere.
Therefore the mass of the additional sulfur or oxygen
increases the mass (and therefore weight) of the coin.
However, the increases are vanishingly small for thin toning
layers, and could not be measured with ordinary equipment.
<<Question: Is the color
progression different for other base metals?
Discussion: Although my sense is they follow the same
general progression, it seems to me that Nickel and Copper
tone a bit differently than silver -- for instance yellows
and blues are much more prevalent on nickel than silver or
copper -- yet this seems at odds with my understanding of
thin film interference.
Does the different underlying color of the natural metal
come in to play here? Is it some other feature of the metals
(perhaps the nature of their oxides and sulfides)? Or is
there something else I'm missing?>>
Other metals will exhibit differences in the color
progression. Copper will be noticeably different due to its
own color. Our perception of color does not reflect
something inherent in a substance, but rather its ability to
absorb light at various frequencies. Even paint pigments
appear the way they do for this reason. In other words,
yellow paint is not inherently yellow; rather it is its
ability to absorb blue light that makes it appear yellow
when illuminated by full-spectrum white light. Copper
appears red-orange because of its ability to absorb some
blue and green light. Therefore, the reflected light from a
copper surface will not have all the same wavelengths
present as light reflected of a bright silver surface. This
in turn affects the interference progression. There will
still be a thin film phenomenon, and a color progression,
but somewhat modified from the one for silver coins.
On nickel I would expect the progression to be closer to
that of silver, but I have not studied enough toned nickel
to verify this.
My observation & toning classification system were intended
only for use on silver coins, at least for now.
<<I have a problem/question with all of
this. For this to be true, one must assume that a coin's
surface is quite smooth. However, my understanding is that a
freshly minted coin's surface has many minute ridges from
the stretching of metal when the coin is struck. These
micro-ridges produce the shimmer we call luster. When one
dips a coin, these ridges are reduced and obliterated to
form a more smooth surface. The same thing happens from
handling, causing a coin to lose it's luster with increased
handling. Coins which have been polished or dipped often
develop colorful toning because of the artificially
flattened contour of the coin's surface. Likewise, proof
coins often show more rapid and pronounced toning than MS
coins.
Assuming the above to be true, the undulating surface of an
uncirculated MS coin with a uniform layer of oxide should
refract a variety of colors because the angle of incidence
of light is changing over the micro-contour or microscopic
surface relief of the coin. Therefore, one should see a mix
of colors (with a prevailing color reflecting/refracting
directly back at the viewer), and a variety of color
transitions with increased depth of the oxidized layer and
the surface topography of the coin. A small point on the
coin should change color as the coin or light is moved.
I say this because I'm trying to make sense out of a very
dark coin I have (Lincoln commemorative half) that shows
rich color when I tilt it to directly reflect light into my
eye. The colors are a deep coppery russet toning with dark
blues, sky blue, yellow, orange, and magenta under intense
light and magnification (7-45X). This color gives way to
glossy black around the lettering. Held in hand under normal
lighting and the coin looks dark brown to coppery colored
unless tilted properly into bright light. >>
Excellent questions and observations. There actually is a
scientific answer to the points you raise, although it took
me a while to figure it out. First, notice in this diagram
that the angle of incidence of ray C is much closer to 90°
than the angle of incidence of Ray B:

The reason the angle of incidence changes is
the difference in the index of refraction between air and
the toning layer. The much denser toning layer permits
transmission of light, but causes the ray to bend when it
enters. As a result, the angles of incidence of all rays
that penetrate the toning layer fall into a narrower range
of angles, closer to 90°. Therefore the extra distance
traveled d=2t/sin(x) stays relatively close to 2t, as the
value of sin(x) is relatively close to 1 for values near
90°. This means that for light penetrating the toning layer,
tilting the angle matters less than you would think. Also
remember that while there may be a bunch of rays with
different trajectories, your eye perceives only the
statistical aggregate of them, the peak of the bell curve
distribution.
As to coins that have a layer of "old time" brownish toning,
that suddenly come to life with reflected colors at just the
correct angle, I think there is an explanation for that
also. I suspect that for some toning layers, the surface
density and refractive index of the toning makes it
difficult for much light to penetrate, unless the angle of
incidence is close to 90°, or at least greater than some
threshold angle x. So if you tilt the coin too far, then the
incident rays come in too far away from a perpendicular
angle to the surface, so most of the light gets absorbed by
the toning layer, or reflected off the upper surface (ray B)
and does not penetrate and reflect (thus no ray C). The
result is that you only see the brownish black color of the
toning layer. However, when you tilt the coin just right,
the angle of incidence gets above the threshold angle,
closer to 90°, and then the light can penetrate and reflect
(ray C), and you see the underlying colors produced by the
thin film effect.
Article by: Doug Kurz (dkurz@mindspring.com)
Compiled and by Brandon Kelley (brandon@jhonecash.com)
Edited and reprinted here from Doug Kurz's
Article on the PCGS message boards with the permission of
Doug Kurz.
|




































